stoichiometric calculation
R23.00
Resource Description
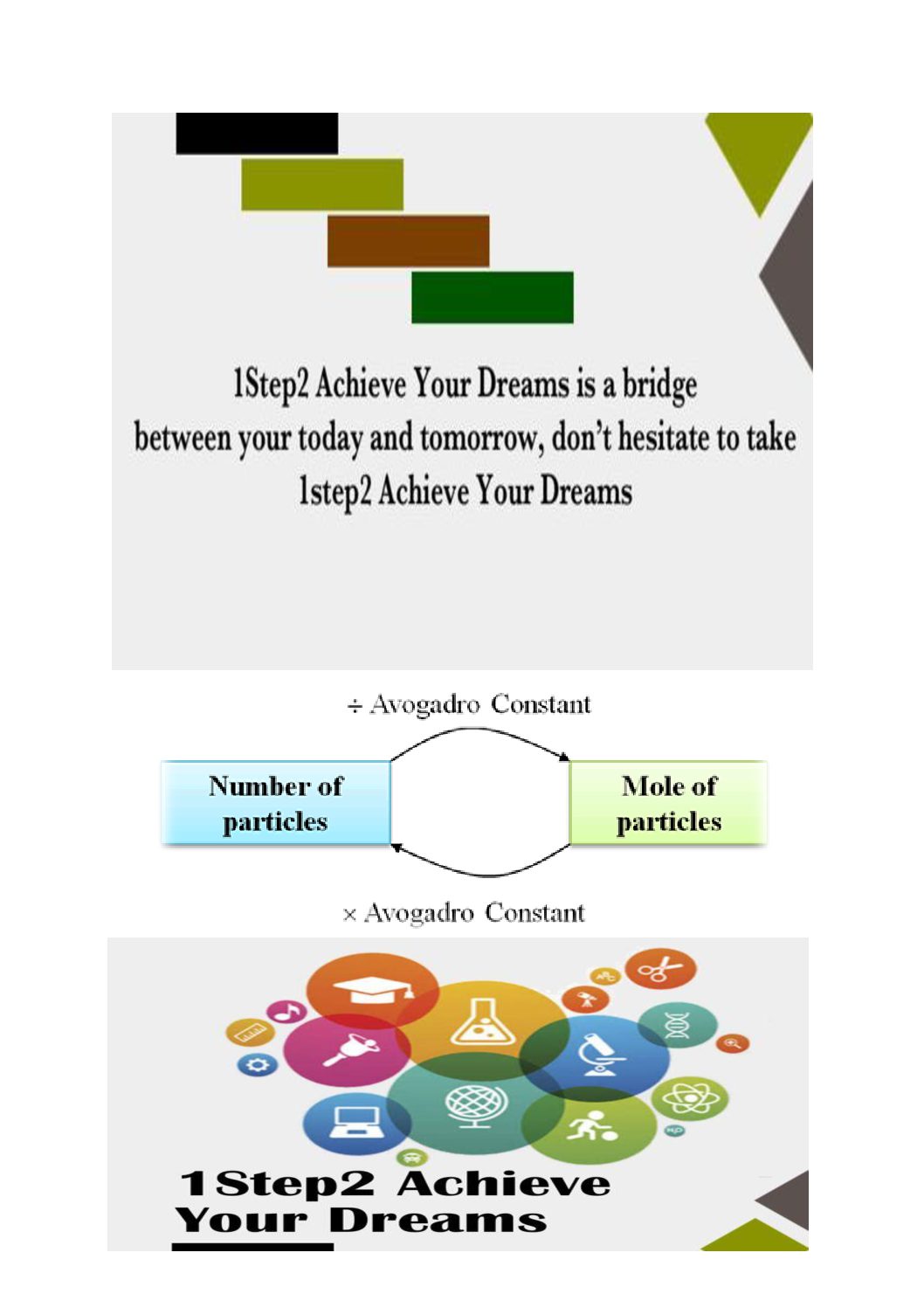
Mass of any atom can be expressed as the relative atomic mass in units of amu. Amu is the atomic mass unit which is 1/12 the mass of one carbon-12 atom. Thus, the mass of an atom in comparison to the carbon-12 atom is called relative atomic mass, amu or u is used as the unit. However, using mass of a single atom is not practical since atoms have so small masses. We rather use mass of a bunch of atoms which will be explained as mole.



 KES(KSh)
KES(KSh) USD($)
USD($) GBP(£)
GBP(£) GHS(₵)
GHS(₵) NGN(₦)
NGN(₦) MUR(₨)
MUR(₨) BWP(P)
BWP(P) AUD($)
AUD($) TZS(Sh)
TZS(Sh) INR(₹)
INR(₹) PHP(₱)
PHP(₱) AED(د.إ)
AED(د.إ)