Grade 12 Chemical equilibrium in PowerPoint
R15.18
Use, by you or one client, in a single end product which end users are not charged for. The total price includes the item price and a buyer fee.
Resource Description
- Open & closed systems
- Concept of dynamic equilibrium
- Phase equilibrium for water
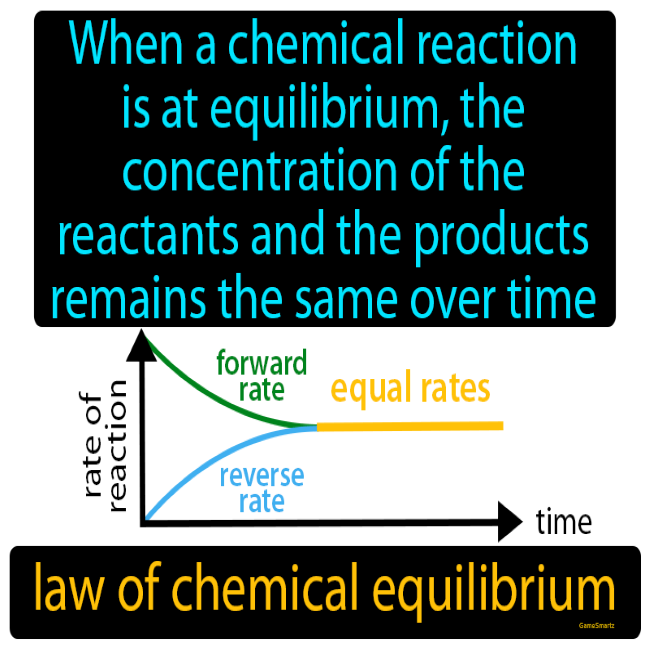
- Basic chemical equilibrium as a concept
- Forward and reverse reaction rates and how they change as equilibrium is established
- Reversible chemical reactions
- Endothermic & exothermic reactions, their rates & graphs & how they change as equilibrium is established
- Explaining chemical equilibrium & the changes that take place in reaction rates before, during and after equilibrium is established.
- Using arrow lengths to help explain and understand reaction rates and how they change
- Graphs – how established and how they change in chemical equilibrium reactions
- Name the factors that affect equilibrium
- Ask yourself: 1. is system closed? 2. is process reversible? 3. is equilibrium dynamic?



 KES(KSh)
KES(KSh) USD($)
USD($) GBP(£)
GBP(£) GHS(₵)
GHS(₵) NGN(₦)
NGN(₦) MUR(₨)
MUR(₨) BWP(P)
BWP(P) AUD($)
AUD($) TZS(Sh)
TZS(Sh) INR(₹)
INR(₹) PHP(₱)
PHP(₱) AED(د.إ)
AED(د.إ)