Grade 12 Galvanic and electrolytic cells in PowerPoint
R15.18
Use, by you or one client, in a single end product which end users are not charged for. The total price includes the item price and a buyer fee.
Resource Description
- Difference between galvanic & electrolytic cells
- Redox reactions
- Oxidation and reduction
- Oxidizing and reducing agents & how to tell the difference
- Balancing equations using half reactions
- Coupling oxidation/reduction reactions
- Direct electron flow
- Copper/silver redox reaction demo
- Components of electrochemical cell
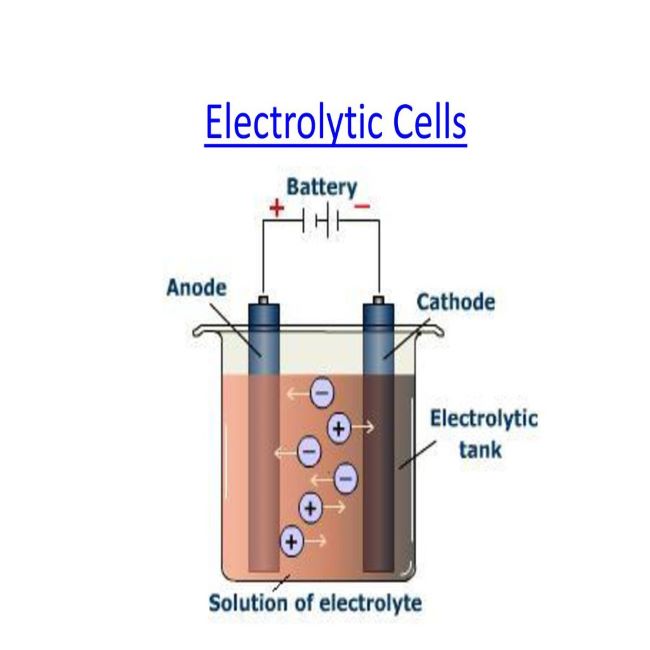
- Anode & cathode & what happens at each.
- Cell notation for an electrochemical cell
- Electrolytic cells & how they operate
- Chemical reactions in cell at electrodes
- Table showing the similarities & differences between these 2 cells.



 KES(KSh)
KES(KSh) USD($)
USD($) GBP(£)
GBP(£) GHS(₵)
GHS(₵) NGN(₦)
NGN(₦) MUR(₨)
MUR(₨) BWP(P)
BWP(P) AUD($)
AUD($) TZS(Sh)
TZS(Sh) INR(₹)
INR(₹) PHP(₱)
PHP(₱) AED(د.إ)
AED(د.إ)