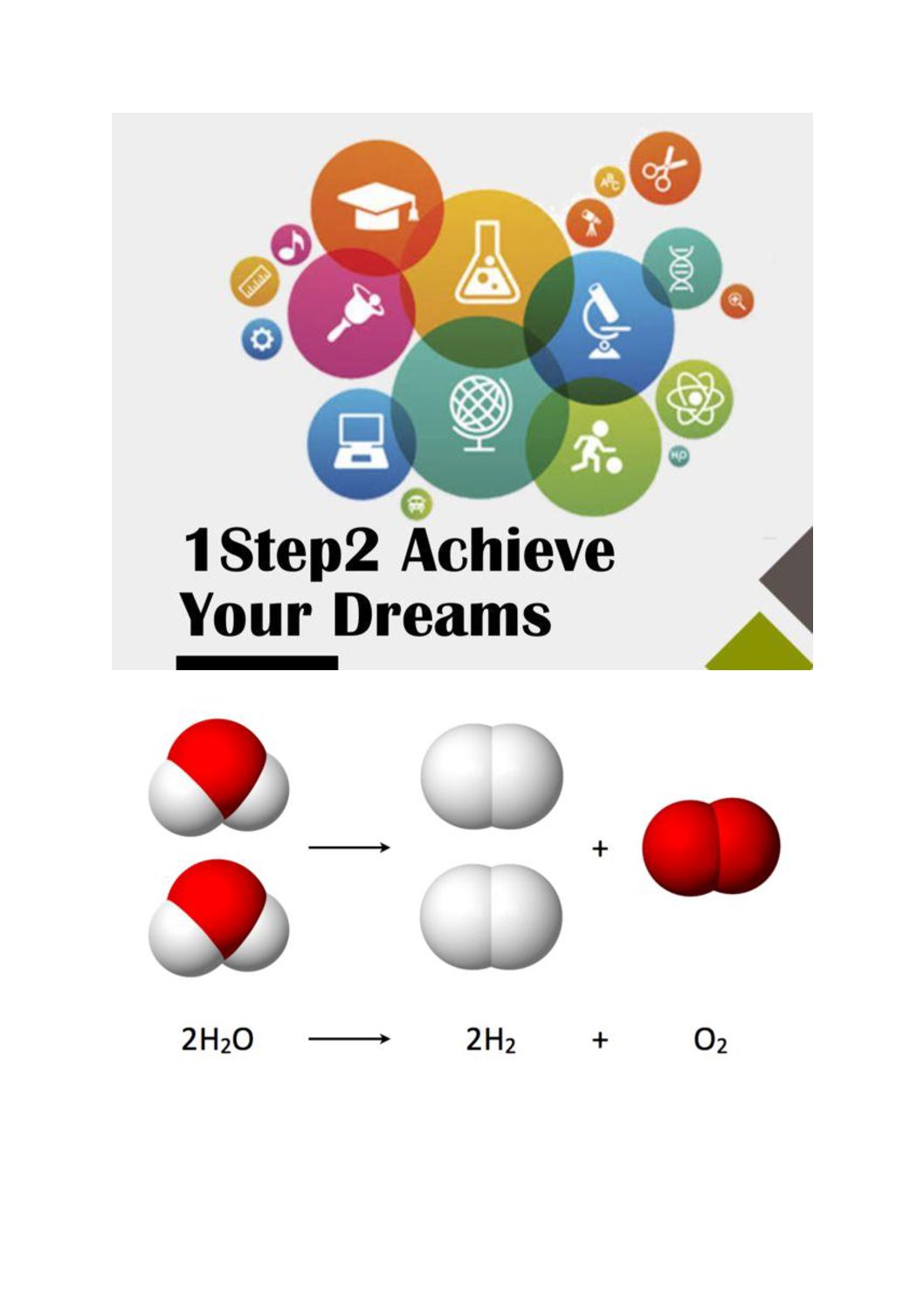
VSEPR Theory & Molecular Shapes
R20.70
Resource Description
in this part of the chapter, we will talk about shape of molecules by using VSEPR theory.
Lewis dot diagram just show us bond between atoms, but do not tell us about shape of molecules.
Covalent bonding forms molecules with specific three-dimensional shapes.
The shape of a molecule depends on the number of electron pairs and lone pairs surrounding the central atom.
One way to predict the geometry of a molecule is to start with the Lewis Dot Diagram, if we look at the number of electron pairs that surround the central atom of molecule, this electron pairs are known as structural electron pairs. The number of structural electron pairs determines the electron pair arrangement and it is telling us the molecular shape of molecules.



 KES(KSh)
KES(KSh) USD($)
USD($) GBP(£)
GBP(£) GHS(₵)
GHS(₵) NGN(₦)
NGN(₦) MUR(₨)
MUR(₨) BWP(P)
BWP(P) AUD($)
AUD($) TZS(Sh)
TZS(Sh) INR(₹)
INR(₹) PHP(₱)
PHP(₱) AED(د.إ)
AED(د.إ)