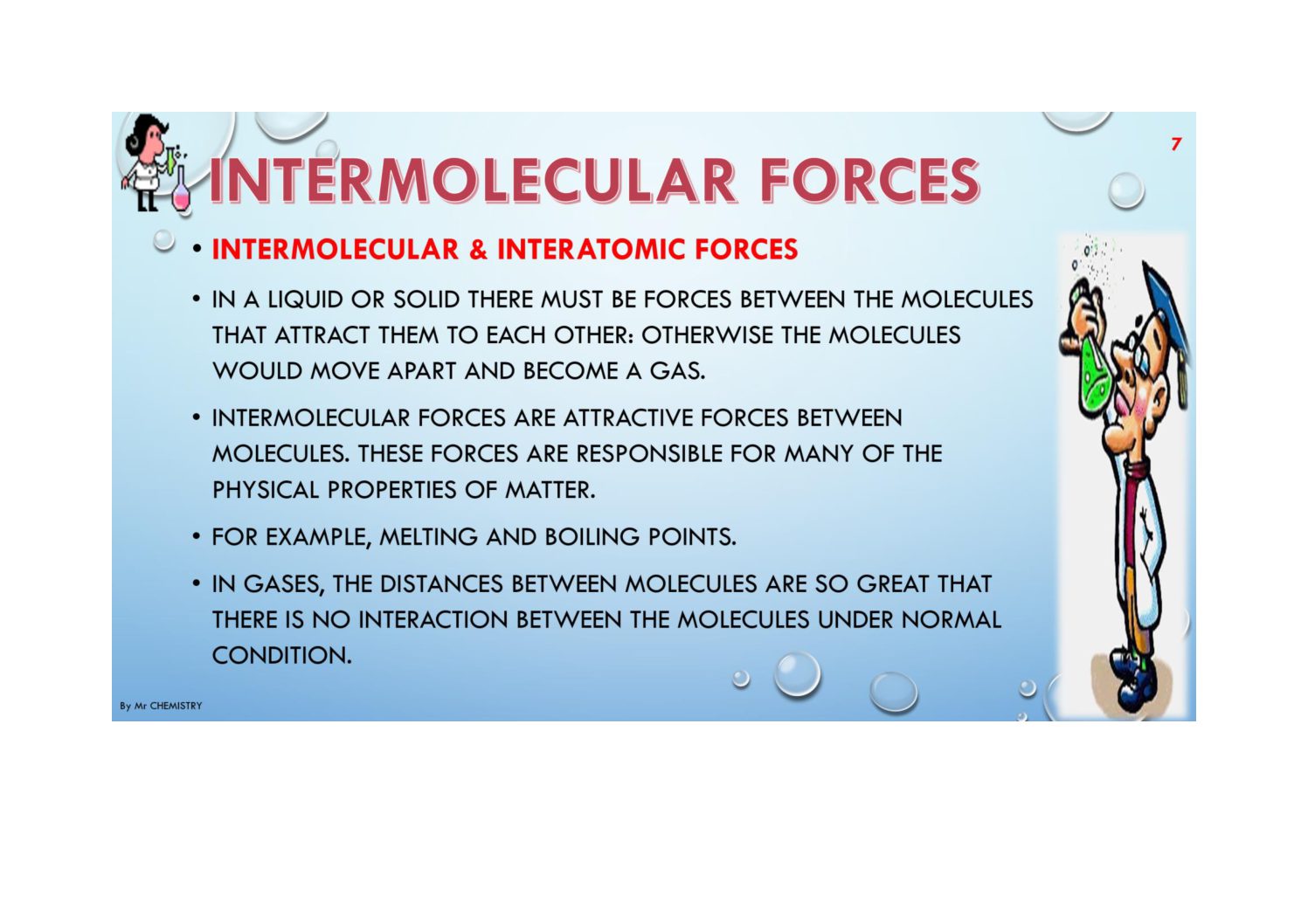
Intermolecular forces
$1
Resource Description
Both the shape of a molecule and the polarity of the bonds determine whether the molecule will be polar or non-polar. Diatomic molecules that contain atoms of the same element are non-polar molecules (for example, Cl2). Diatomic molecules that contain atoms of different elements (and different EN) form polar molecules (for example, HCl).



 KES(KSh)
KES(KSh) USD($)
USD($) GBP(£)
GBP(£) GHS(₵)
GHS(₵) NGN(₦)
NGN(₦) MUR(₨)
MUR(₨) BWP(P)
BWP(P) AUD($)
AUD($) TZS(Sh)
TZS(Sh) INR(₹)
INR(₹) PHP(₱)
PHP(₱) AED(د.إ)
AED(د.إ)