Grade 12 Factors affecting chemical equilibrium in PowerPoint
$1
Use, by you or one client, in a single end product which end users are not charged for. The total price includes the item price and a buyer fee.
Resource Description
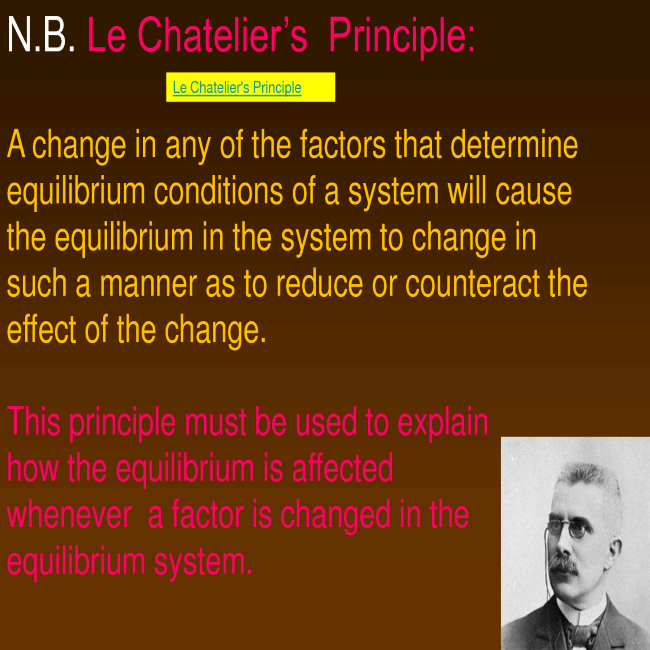
- Le Chatelier’s principle
- Change in concentration and how it affects equilibrium
- Change in pressure & how it affects equilibrium
- Change in temperature & how it affects equilibrium
- Using a catalyst and equilibrium
- How equilibrium is affected in these changes
- How equilibrium constant is affected with these changes
- How equilibrium graphs are affected by these changes
- Haber process for the preparation of ammonia, using changes in factors & affecting equilibrium
- Contact process for preparation of sulphuric acid in industry
- The common ion effect
- Summary & comparison table indicating change, system response to change & change in equilibrium constant, or not.



 KES(KSh)
KES(KSh) USD($)
USD($) GBP(£)
GBP(£) GHS(₵)
GHS(₵) NGN(₦)
NGN(₦) MUR(₨)
MUR(₨) BWP(P)
BWP(P) AUD($)
AUD($) TZS(Sh)
TZS(Sh) INR(₹)
INR(₹) PHP(₱)
PHP(₱) AED(د.إ)
AED(د.إ)