Grade 11 Redox reactions in PowerPoint.
$1
Use, by you or one client, in a single end product which end users are not charged for. The total price includes the item price and a buyer fee.
Resource Description
- Burning Mg
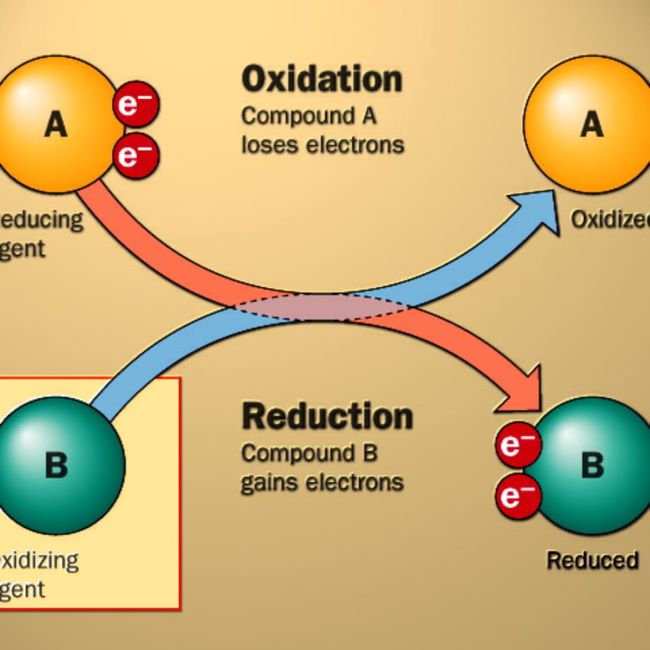
- Oxidation & reduction half reactions
- OILRIG – Oxidation Is Loss & Reduction Is Gain of electrons
- Identification of substances oxidized & reduced
- Oxidation numbers & their use
- Rules for allocation of oxidation numbers
- Many examples
- Oxidation numbers in compounds and reactions.



 KES(KSh)
KES(KSh) USD($)
USD($) GBP(£)
GBP(£) GHS(₵)
GHS(₵) NGN(₦)
NGN(₦) MUR(₨)
MUR(₨) BWP(P)
BWP(P) AUD($)
AUD($) TZS(Sh)
TZS(Sh) INR(₹)
INR(₹) PHP(₱)
PHP(₱) AED(د.إ)
AED(د.إ)